The earliest metallurgical process for treating silver ores was amalgamation with mercury which was in use in the early 1500's. Closely following was the development of the Patio process for treating ores at Pachuca, Mexico.
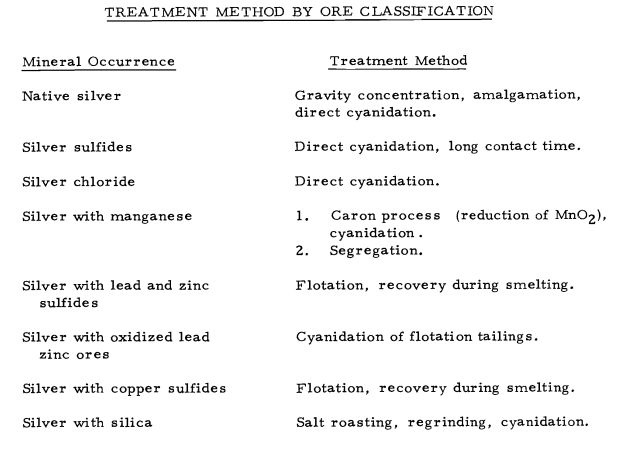
Silver often occurs as the native metal and in deposits associated with other metals such as gold, copper, lead and zinc. Principal silver minerals include compounds of sulfur, antimony, arsenic, and copper. Silver chloride, and argentiferous galena are also prime sources.
Native silver and the chloride characterize the oxide zone of most deposits. Native silver can be concentrated, amalgamated, or cyanided. Silver chloride rapidly dissolves in cyanide without oxygen. Oxidized silver ores containing the higher oxides of manganese are generally refractory to metallurgical treatment. A refractory compound of manganese and silver is formed, probably a manganite, which is insoluble in cyanide solutions.
Argentite is the predominant silver mineral. Other important economic minerals are native silver, argentiferous galena, cerargyrite, pyrargyrite and tetrahedrite. Argentite dissolves slowly, the reaction being reversible requires an excess of cyanide. Argentite with disseminated fine grained gold occurring in quartz veins with minor amounts of chalcopyrite and galena may be cyanided directly after grinding, with high silver and gold recoveries. The flowsheet adapted to this type of ore is the Minas de San Luis, Tayoltita cyanide plant, which treats a high grade argentite silver ore containing gold. It is a standard cyanidation circuit but employs long periods of agitation contact for the high silver sulfide content ore.
Lead-zinc base metal semi-oxidized-complex ores in pipes and chimneys are replacement deposits in limestone at El Mochito Mine, Honduras. Mineralization comprises galena, cerussite, anglesite, sphalerite, calamine, and smithsonite. The silver content of about 16 ounces occurs as native silver globs, wire and the mineral argentite. After flotation of lead and zinc concentrates, the tailings are cyanided for gold and silver recovery.
The great Huelva pyritic copper deposits of Spain carry a little gold and silver. At Cerro Colorado, these metals were concentrated above the chalcocite zone into the so-labeled "gossan". This material with a content of 0. 08-0. 09 oz Au and 1.50 oz Ag per ton is amenable to cyanidation.
See Cerro Colorado flowsheet.
In southern Hidalgo, about 60 miles north of Mexico City, are the great silver--gold veins of one of the foremost silver mining districts of the world. Real del Monte, Pachuca. The mineralized area consists of flow rocks and intrusives. The oxidized ores carry pyrites with oxides of iron and manganese and other minerals, silver and gold. The first zone contains auriferous iron oxide, chlorides, and bromides of silver. These ores can be cyanided directly. The lower zone contains pyrite, galena, sphalerite, argentite, and chalcopyrite. The Del Monte flowsheet uses selective flotation to produce a lead, zinc, and pyrite concentrate. The tailings are cyanided.
Silver often occurs as the native metal and in deposits associated with other metals such as gold, copper, lead and zinc. Principal silver minerals include compounds of sulfur, antimony, arsenic, and copper. Silver chloride, and argentiferous galena are also prime sources.
Native silver and the chloride characterize the oxide zone of most deposits. Native silver can be concentrated, amalgamated, or cyanided. Silver chloride rapidly dissolves in cyanide without oxygen. Oxidized silver ores containing the higher oxides of manganese are generally refractory to metallurgical treatment. A refractory compound of manganese and silver is formed, probably a manganite, which is insoluble in cyanide solutions.
Argentite is the predominant silver mineral. Other important economic minerals are native silver, argentiferous galena, cerargyrite, pyrargyrite and tetrahedrite. Argentite dissolves slowly, the reaction being reversible requires an excess of cyanide. Argentite with disseminated fine grained gold occurring in quartz veins with minor amounts of chalcopyrite and galena may be cyanided directly after grinding, with high silver and gold recoveries. The flowsheet adapted to this type of ore is the Minas de San Luis, Tayoltita cyanide plant, which treats a high grade argentite silver ore containing gold. It is a standard cyanidation circuit but employs long periods of agitation contact for the high silver sulfide content ore.
Lead-zinc base metal semi-oxidized-complex ores in pipes and chimneys are replacement deposits in limestone at El Mochito Mine, Honduras. Mineralization comprises galena, cerussite, anglesite, sphalerite, calamine, and smithsonite. The silver content of about 16 ounces occurs as native silver globs, wire and the mineral argentite. After flotation of lead and zinc concentrates, the tailings are cyanided for gold and silver recovery.
The great Huelva pyritic copper deposits of Spain carry a little gold and silver. At Cerro Colorado, these metals were concentrated above the chalcocite zone into the so-labeled "gossan". This material with a content of 0. 08-0. 09 oz Au and 1.50 oz Ag per ton is amenable to cyanidation.
See Cerro Colorado flowsheet.
In southern Hidalgo, about 60 miles north of Mexico City, are the great silver--gold veins of one of the foremost silver mining districts of the world. Real del Monte, Pachuca. The mineralized area consists of flow rocks and intrusives. The oxidized ores carry pyrites with oxides of iron and manganese and other minerals, silver and gold. The first zone contains auriferous iron oxide, chlorides, and bromides of silver. These ores can be cyanided directly. The lower zone contains pyrite, galena, sphalerite, argentite, and chalcopyrite. The Del Monte flowsheet uses selective flotation to produce a lead, zinc, and pyrite concentrate. The tailings are cyanided.
 RSS Feed
RSS Feed
